
For this month’s column, I knew that I wanted to write about the 125th anniversary of the electron’s discovery, which for simplicity’s sake I pegged to Thomson’s lecture. Of course, history is always more muddled than that. Thomson is often hailed as the discoverer of the electron based on that lecture 125 years ago. Corpuscles are electrons, and the plum pudding model gave way to Ernest Rutherford’s nuclear model in 1911. Thomson, who merely endorsed the idea.Ĭorpuscles and pudding are not how we think about the structure of an atom today. The model also became known as the Thomson model, although its chief proponent was William Thomson (Lord Kelvin), not J.J. In Thomson’s analogy, negatively charged corpuscles were like raisins suspended in a positively charged cake, resulting in a neutral atom. This model of the atom became known as the “plum pudding” model, so named for the popular English dessert. Thomson described his experiments with cathode rays to verify the existence of these subatomic corpuscles. “The atoms of the ordinary elements are made up of corpuscles and holes, the holes being predominant,” he continued. Thomson, during a lecture at the Royal Institution in London, on 30 April 1897.

The value was found to be 9.11 × 10 -10 gĪn electron is that fundamental particles which carries 1 unit negative charge and has a mass nearly equal to 1/1837 th of that hydrogen atom.“We shall call such particles corpuscles,” announced the physicist J.J.
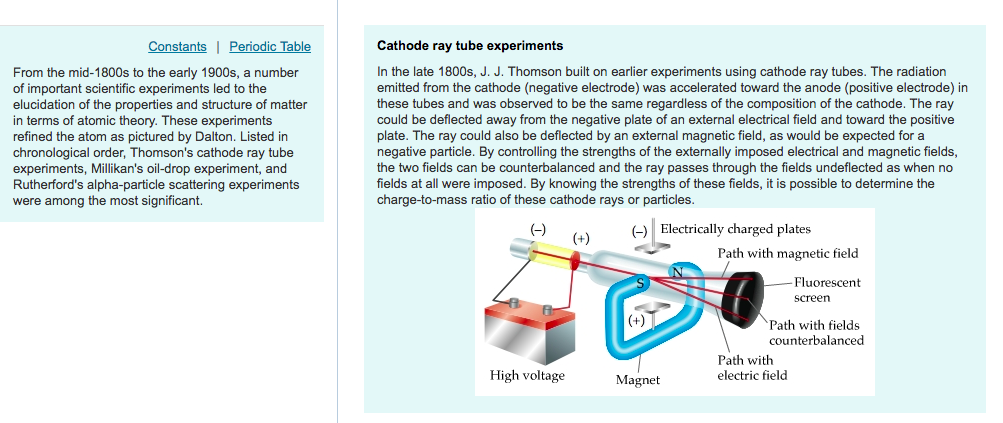
Millikan in 1917 with the help of his Oil Drop experiment. The charge on the electron was found by R.A. The value was found to be 1.76 × 10 8 coulombs/ gįor the determination of the charge on the electron He found every time that the ratio of charge/mass of the electron was the same. He used different discharge tubes fitted with electrodes of different metals. The negatively charged material particles constituting the cathode rays are called electrons.įor determination of the ratio of charge/mass of electrons This indicates that cathode ray produce heating effect.ĥ)They produce green fluorescence on the glass walls of the discharge tube as well as on certain other fluorescent substances such as zinc sulphide.Ħ)They possess penetrating effect ie they can easily pass through thin foils of metals.

This shows that cathode rays carry negative charge.Ĥ)When cathode rays strike a metal foil, the latter becomes hot. This shows that cathode rays are made up of material particles.ģ)When an electric field is applied on the cathode rays, they are deflected towards the positive plate of the electric field. This shows that cathode rays travel in straight line.Ģ)If a light paddle wheel mounted on an axle is placed in their path, the wheel begins to rotate.
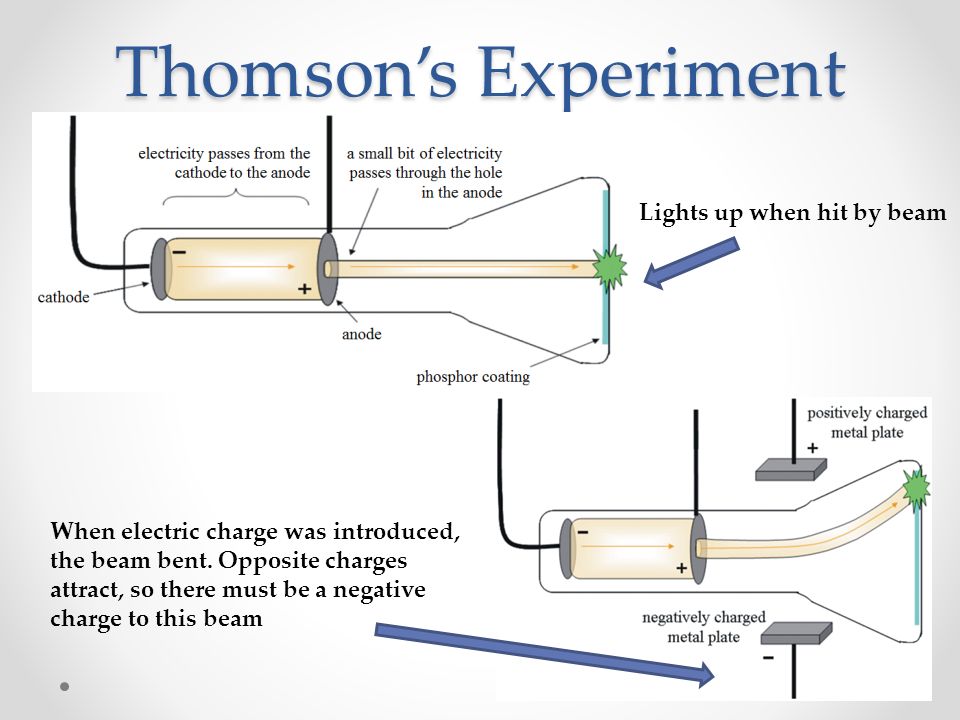
These rays were called as cathode rays.ġ)They produce a sharp Shadow of the solid object placed in their path. This shows that some invisible rays are emitted from the cathode which passed through the holes of the anode and strike the wall. 3) When the pressure is for the reduced to about 0.01 millimetre, the glow between the electrodes disappear but the current continuous to flow and if a perforated anode is used, a faint greenish glow is observed on the glass walls behind the anode.


 0 kommentar(er)
0 kommentar(er)
